About MDSSPRO
Home – About Us
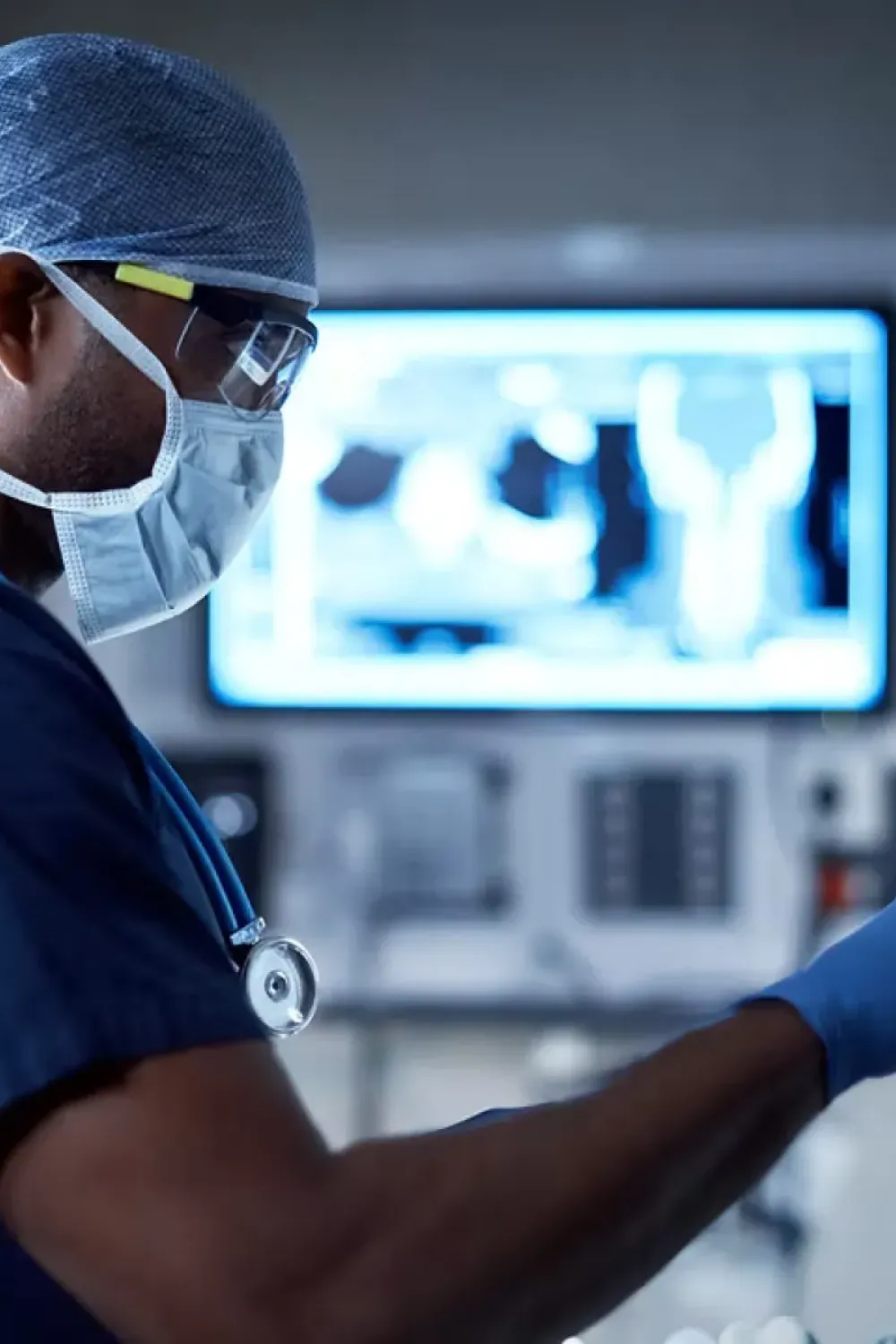
Who Are We?
Specialists in Medical Device Regulation, Training, and Compliance
At MDSSPRO (Medical Device Software and Sterilization Professionals), we believe that we can help any company in medical devices to achieve and maintain full compliance with the MDR (Medical Device Regulation) with confidence. MDSSPRO was founded in 2016 by Patricia Vest and is based on over 20 years of experience in auditing and regulatory affairs through working with leading European Notified Bodies, including TÜV SÜD, SGS, UL and Intertek.
We have a simple mission – to help our clients develop a simple understanding, management and implementation of complex requirements under the MDR using our services in specialised training and Auditing. Our team brings technical expertise, industry knowledge and excellence to every engagement.
Industry Experience
We have over 20 years of experience in medical device regulation, auditing and evaluating technical documentation development, so each of our clients benefits from the confidence of accurate, consistent and practical regulatory service.
Specialist Knowledge
All our trainers and auditors are MDR specialists who have extensive hands-on expertise in various technical disciplines such as:
1. Medical device software
2. Sterilization processes
3. Electrical safety
4. Microbiology and biocompatibility
“By working with MDSSPRO, you obtain not only a service provider but a trusted partner in quality and compliance that is dedicated to your long-term success in the medical device sector.”
What do We Do?
We provide Comprehensive MDR Training and Auditing Solutions

MDR Training Tailored for You
Our training courses are tailor-made to suit your organisation's size, product range, and compliance targets. Whether on-site, facilitating a short workshop, on a one-to-one basis, or conducting webinars for larger teams, we offer a flexible and engaging learning experience which has tangible outcomes.

"Expert" Auditing Services
Our auditors are highly experienced, bringing over 20 years of industry and field experience, carrying out internal and supplier audits to ISO 13485:2016, UK MDR, and MDR 2017/745. Additionally, we provide preparation and support with Notified Body audits, which helps clients enhance their quality management system and documentation before their audits.
Learning Online & On-Site
We recognise that each organisation learns differently; therefore, we offer flexible delivery through personalised online sessions, and on themed workshops and seminars.

Why Choose MDSSPRO?
Subject Matter Experts in Medical Device Regulation and Compliance
Partnering with MDSSPRO indicates that you will work with an organisation built on integrity, flexibility, and client satisfaction.
We are pleased to offer training and auditing services that are:
- Experienced and Trusted: 20+ years of work in both MDR and auditing from respected European Notified Bodies.
- Specialist-led: Trainers with real-life experience regarding software, sterilisation, electrical safety, and microbiology.
- Tailored and Transparent: Training created with your business in mind, with no hidden costs and schedules that can change at short notice depending on your company's needs.
- Value for Money: Outstanding value from training and consultancy designed to achieve the best results.
Clients continue to trust us because we provide technical rigour with a personal, outcomes-based approach.
What Our Clients Say?
Read Positive Stories of Change
We are pleased that we receive positive testimonials from our clients who have benefited from our training and auditing practice.
Our team will remain motivated to leave each delegate feeling competent and confident of what they have learned, as well as understanding how they can apply MDR working practices to their working lives.
The tutors are true experts with extensive knowledge of MDR and sterilization standards. Their practical approach helped me connect the theory to real-life scenarios in my company. I feel much more confident handling compliance documentation now.
The EO sterilization validation course was very detailed and aligned perfectly with ISO 11135. It gave me a clear understanding of validation, indicators, and regulatory expectations. This training is a must for quality professionals.
Let's Build Compliance Confidence Together
Choose MDSSPRO, and we will ensure your medical devices comply with the highest standards of regulation. Whether we provide training tailored specifically to your organisation or through auditing, we will guide you step-by-step as you navigate change.


The EO sterilization validation course was very detailed and aligned perfectly with ISO 11135. It gave me a clear understanding of validation, indicators, and regulatory expectations. This training is a must for quality professionals.